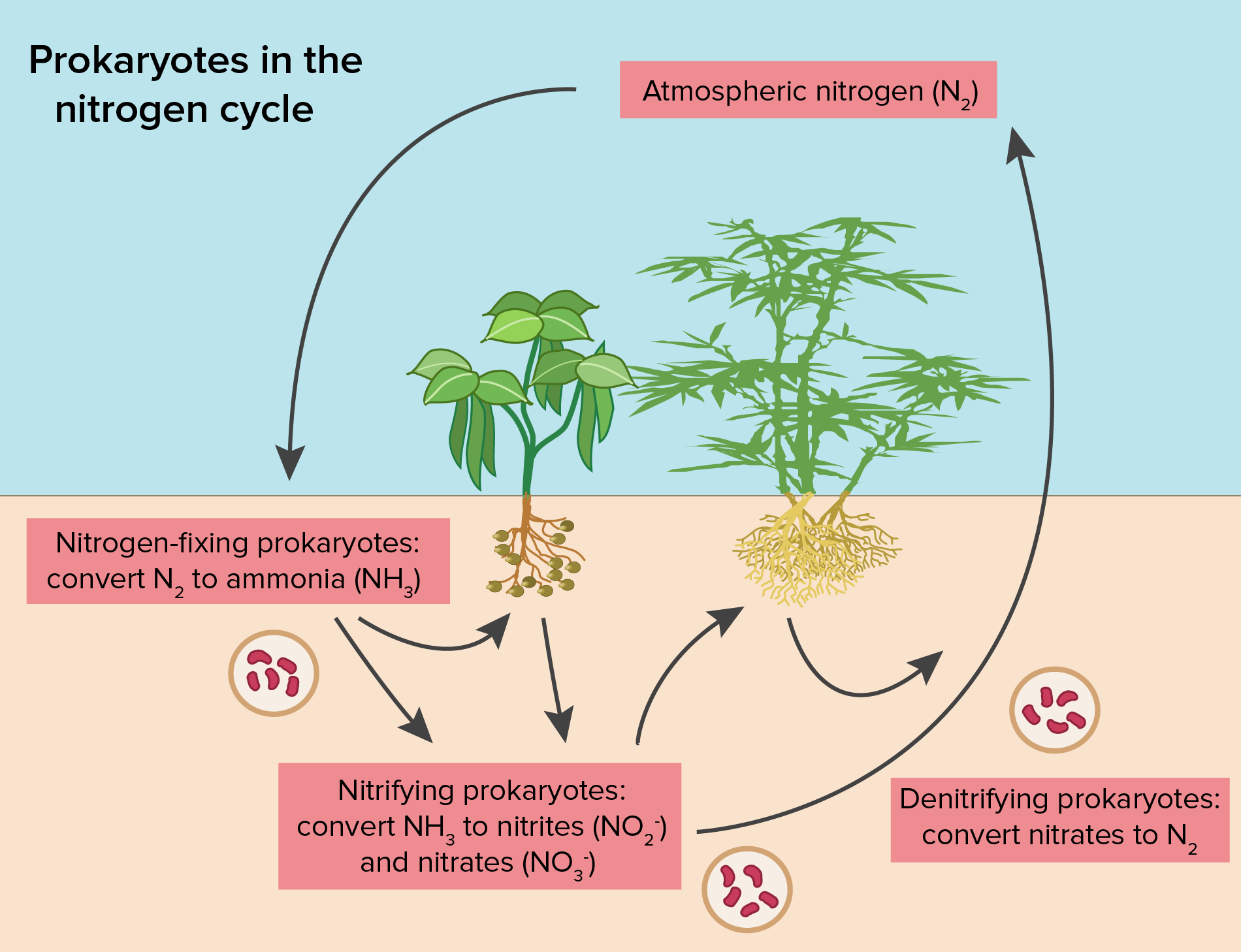
How Does Nitrogen React With Water . The atmosphere consists of 78% nitrogen by volume. It exhibits polarity and is naturally. reactions of main group elements with water. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: nitrogen mainly occurs in wastewater in this form. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). most pure nitrogen comes from the fractional distillation of liquid air. Nitric oxide is more active. Under some conditions these complexes. After biological wastewater treatment, it mainly occurs as oxidized nitrite. Water is composed of two hydrogen atoms and an oxygen atom.
from rachel-yersblogdalton.blogspot.com
nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). most pure nitrogen comes from the fractional distillation of liquid air. After biological wastewater treatment, it mainly occurs as oxidized nitrite. It exhibits polarity and is naturally. reactions of main group elements with water. nitrogen mainly occurs in wastewater in this form. Nitric oxide is more active. Water is composed of two hydrogen atoms and an oxygen atom. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature:
Explain Different Steps of Nitrogen Cycle
How Does Nitrogen React With Water reactions of main group elements with water. most pure nitrogen comes from the fractional distillation of liquid air. Under some conditions these complexes. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: nitrogen mainly occurs in wastewater in this form. The atmosphere consists of 78% nitrogen by volume. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: Nitric oxide is more active. Water is composed of two hydrogen atoms and an oxygen atom. nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). After biological wastewater treatment, it mainly occurs as oxidized nitrite. reactions of main group elements with water. It exhibits polarity and is naturally.
From open.oregonstate.education
Chemolithotrophy & Nitrogen Metabolism General Microbiology How Does Nitrogen React With Water It exhibits polarity and is naturally. Under some conditions these complexes. After biological wastewater treatment, it mainly occurs as oxidized nitrite. nitrogen mainly occurs in wastewater in this form. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: the electrical energy from either of those sources causes nitrogen and oxygen to. How Does Nitrogen React With Water.
From socratic.org
How many grams of nitrogen dioxide must react with water to produce 5.00*10^22 molecules of How Does Nitrogen React With Water Nitric oxide is more active. The atmosphere consists of 78% nitrogen by volume. reactions of main group elements with water. It exhibits polarity and is naturally. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: After biological wastewater treatment, it mainly occurs as oxidized nitrite. nitrogen forms complexes with transition. How Does Nitrogen React With Water.
From www.earth.com
Why is the Nitrogen Cycle So Important? How Does Nitrogen React With Water reactions of main group elements with water. Nitric oxide is more active. most pure nitrogen comes from the fractional distillation of liquid air. Water is composed of two hydrogen atoms and an oxygen atom. After biological wastewater treatment, it mainly occurs as oxidized nitrite. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at. How Does Nitrogen React With Water.
From www.earth.com
Why is the Nitrogen Cycle So Important? How Does Nitrogen React With Water Nitric oxide is more active. After biological wastewater treatment, it mainly occurs as oxidized nitrite. most pure nitrogen comes from the fractional distillation of liquid air. nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: reactions of main group. How Does Nitrogen React With Water.
From mavink.com
Wetland Nitrogen Cycle How Does Nitrogen React With Water It exhibits polarity and is naturally. reactions of main group elements with water. most pure nitrogen comes from the fractional distillation of liquid air. After biological wastewater treatment, it mainly occurs as oxidized nitrite. Under some conditions these complexes. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: The atmosphere consists. How Does Nitrogen React With Water.
From slideplayer.com
Water cycle Carbon cycle Nitrogen cycle Phosphorus cycle ppt download How Does Nitrogen React With Water Under some conditions these complexes. After biological wastewater treatment, it mainly occurs as oxidized nitrite. nitrogen mainly occurs in wastewater in this form. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: Water is composed of two hydrogen atoms and an oxygen atom. It exhibits polarity and is naturally. again. How Does Nitrogen React With Water.
From www.researchgate.net
Biological nitrogen removal reactions a) Conventional biological... Download Scientific Diagram How Does Nitrogen React With Water The atmosphere consists of 78% nitrogen by volume. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: Under some conditions these complexes. reactions of main group elements with water. Nitric oxide is more active. After biological wastewater treatment, it mainly occurs as oxidized nitrite. It exhibits polarity and is naturally. the. How Does Nitrogen React With Water.
From www.youtube.com
How to Balance NO2 + H2O = HNO2 + HNO3 (Nitrogen dioxide + Water) YouTube How Does Nitrogen React With Water nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). Nitric oxide is more active. After biological wastewater treatment, it mainly occurs as oxidized nitrite. Under some conditions these complexes. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: nitrogen mainly occurs in wastewater in this form. the electrical energy. How Does Nitrogen React With Water.
From www.science-sparks.com
What is the Nitrogen Cycle? Science for Kids How Does Nitrogen React With Water Under some conditions these complexes. Nitric oxide is more active. most pure nitrogen comes from the fractional distillation of liquid air. reactions of main group elements with water. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: After biological wastewater treatment, it mainly occurs as oxidized nitrite. nitrogen forms. How Does Nitrogen React With Water.
From nitrogengassanroso.blogspot.com
Nitrogen Gas Nitrogen Gas And Oxygen Gas Reaction How Does Nitrogen React With Water again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: The atmosphere consists of 78% nitrogen by volume. Water is composed of two hydrogen atoms and an oxygen atom. nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). the electrical energy from either of those sources causes nitrogen and oxygen to. How Does Nitrogen React With Water.
From water.unl.edu
Nitrogen Dynamics UNL Water How Does Nitrogen React With Water It exhibits polarity and is naturally. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: nitrogen mainly occurs in wastewater in this form. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: Nitric oxide is more active. The atmosphere consists of 78% nitrogen by. How Does Nitrogen React With Water.
From slidetodoc.com
The Nitrogen Cycle Nitrogen like water is recycled How Does Nitrogen React With Water nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). Water is composed of two hydrogen atoms and an oxygen atom. The atmosphere consists of 78% nitrogen by volume. reactions of main group elements with water. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: most pure nitrogen comes from. How Does Nitrogen React With Water.
From www.youtube.com
A2 Biology Reactions in the nitrogen cycle (OCR A Chapter 23.3) YouTube How Does Nitrogen React With Water reactions of main group elements with water. Water is composed of two hydrogen atoms and an oxygen atom. most pure nitrogen comes from the fractional distillation of liquid air. nitrogen mainly occurs in wastewater in this form. Under some conditions these complexes. nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). Nitric oxide is more. How Does Nitrogen React With Water.
From dailysciencejournal.com
Atomic Number 7 A Quick Guide to Nitrogen Daily Science Journal How Does Nitrogen React With Water the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: After biological wastewater treatment, it mainly occurs as oxidized nitrite. most pure nitrogen comes from the fractional distillation of liquid air. It exhibits polarity and is naturally. nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). Nitric oxide is more. How Does Nitrogen React With Water.
From mavink.com
Nitrogen And Hydrogen Reaction How Does Nitrogen React With Water nitrogen mainly occurs in wastewater in this form. the electrical energy from either of those sources causes nitrogen and oxygen to form nitric oxide: Under some conditions these complexes. After biological wastewater treatment, it mainly occurs as oxidized nitrite. Nitric oxide is more active. reactions of main group elements with water. Water is composed of two hydrogen. How Does Nitrogen React With Water.
From rachel-yersblogdalton.blogspot.com
Explain Different Steps of Nitrogen Cycle How Does Nitrogen React With Water The atmosphere consists of 78% nitrogen by volume. nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). Water is composed of two hydrogen atoms and an oxygen atom. Under some conditions these complexes. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: reactions of main group elements with water. . How Does Nitrogen React With Water.
From reefnation.com
The Nitrogen Cycle Revisited How Does Nitrogen React With Water It exhibits polarity and is naturally. Water is composed of two hydrogen atoms and an oxygen atom. Nitric oxide is more active. Under some conditions these complexes. reactions of main group elements with water. nitrogen mainly occurs in wastewater in this form. After biological wastewater treatment, it mainly occurs as oxidized nitrite. most pure nitrogen comes from. How Does Nitrogen React With Water.
From water.mecc.edu
Lesson 21 Nitrogen How Does Nitrogen React With Water Nitric oxide is more active. again in contrast to carbon, nitrogen undergoes only two important chemical reactions at room temperature: After biological wastewater treatment, it mainly occurs as oxidized nitrite. nitrogen forms complexes with transition metals yielding nitrogeno complexes, (8.2.5). nitrogen mainly occurs in wastewater in this form. It exhibits polarity and is naturally. Under some conditions. How Does Nitrogen React With Water.